You Are About to Leave This Site
You will be leaving this Regeneron US website and going to a third-party independent website. Regeneron provides these links for your information only and takes no responsibility for the content on any such website.
As of March 2, 2026
INDICATIONS
EYLEA HD® (aflibercept) Injection 8 mg and EYLEA® (aflibercept) Injection 2 mg are indicated for the treatment of patients with Neovascular (Wet) Age-Related Macular Degeneration (AMD), Diabetic Macular Edema (DME), Diabetic Retinopathy (DR), and Macular Edema following Retinal Vein Occlusion (RVO).
IMPORTANT SAFETY INFORMATION FOR EYLEA HD AND EYLEA
CONTRAINDICATIONS
- EYLEA HD and EYLEA are contraindicated in patients with ocular or periocular infections, active intraocular inflammation, or known hypersensitivity to aflibercept or any of the excipients in EYLEA HD or EYLEA.
Please see additional Important Safety Information below.
EYLEA HD is now approved for MEfRVO and Q4W dosing in appropriate patients for all indications, including Wet AMD, DME, DR, and MEfRVO!1
Recommended dosing:
Wet AMD and DME: 8 mg administered by intravitreal injection Q4W (+/- 7 days) for the first 3 doses, followed by 8 mg via intravitreal injection once every 8 to 16 weeks (+/- 1 week).
DR: 8 mg administered by intravitreal injection Q4W (+/- 7 days) for the first 3 doses, followed by 8 mg via intravitreal injection once every 8 to 12 weeks (+/- 1 week).
MEfRVO: 8 mg administered by intravitreal injection every 4 weeks (+/- 7 days) for the first 3 to 5 doses, followed by 8 mg via intravitreal injection once every 8 weeks (+/- 1 week).
For Wet AMD, DME, and DR: Some patients did not maintain a response with extended dosing intervals after successful response to the 3 initial monthly doses. These patients may benefit from resuming every-4-week dosing (approximately every 28 days +/- 7 days).
For MEfRVO: Some patients did not maintain a response with extended dosing intervals after successful response to the first 3 to 5 initial monthly doses. These patients may benefit from resuming every-4-week dosing (approximately every 28 days +/- 7 days).
EYLEA HD Coverage and Access
100% of eligible patients with Medicare Fee-for-Service (FFS) have first-line access to EYLEA HD with no prior authorization or step edit required2

For an up-to-date payer coverage report for national and regional payers, click here
Please be sure to clear your browser cache and history to view the latest payer coverage report via the link above.
| * | Individual plan policies may vary, and the information presented herein does not replace a benefit verification. All formulary data and other access criteria are provided by Managed Markets Insight & Technology, LLC database as of January 2026 and EYLEA HD imputed sales as of September 2025. |
March 2026 Payer Coverage Alerts
| Health Plan* | Channel | Indication(s) | Status† | Effective Date | Policy Link |
| BCBS of Illinois (HCSC) | Commercial | Wet AMD, DME, DR, MEfRVO | First-line access, no step edit | 01/01/2026 | medicalpolicy.hcsc.com |
| BCBS of Montana (HCSC) | Commercial | Wet AMD, DME, DR, MEfRVO | First-line access, no step edit | 01/01/2026 | medicalpolicy.hcsc.com |
| BCBS of New Mexico (HCSC) | Commercial | Wet AMD, DME, DR, MEfRVO | First-line access, no step edit | 01/01/2026 | medicalpolicy.hcsc.com |
| BCBS of Oklahoma (HCSC) | Commercial | Wet AMD, DME, DR, MEfRVO | First-line access, no step edit | 01/01/2026 | medicalpolicy.hcsc.com |
| BCBS of Texas (HCSC) | Commercial | Wet AMD, DME, DR, MEfRVO | First-line access, no step edit | 01/01/2026 | medicalpolicy.hcsc.com |
| Wellmark | Commercial | Wet AMD, DME, DR, MEfRVO | First-line access, no step edit | 01/01/2026 | wellmark.com |
| BCBS of Massachusetts | Medicare Advantage | Wet AMD, DME, DR, MEfRVO | Single agent step through bevacizumab | 01/01/2026 | bluecrossma.org |
| Excellus (BCBS) | Commercial and Medicare Advantage | Wet AMD, DME, DR, MEfRVO | Single agent step through 3 bevacizumab injections | 01/01/2026 | provider.excellusbcbs.com |
| Excellus (BCBS) | Commercial and Medicare Advantage | DME, DR | 20/50 or worse vision – first-line access, no step edit. Single agent step through bevacizumab if 20/50 or better | 01/01/2026 | provider.excellusbcbs.com |
|
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
|
| * | For plans with step therapy requirements, review your patient’s Summary of Benefits for specific criteria. |
| † | Prior authorization may still be required. |
| AMD = age-related macular degeneration; DME = diabetic macular edema; DR = diabetic retinopathy; MEfRVO = macular edema following retinal vein occlusion. | |
| All formulary data and other access criteria are provided by Managed Markets Insight & Technology, LLC database as of January 2026. | |
| Individual plan policies may vary, and the information presented herein does not replace a benefit verification. |
Medicare in Focus
Our Medicare in Focus initiative provides patients with information and education on the differences between Original Medicare and Medicare Advantage to support their selection of the most appropriate coverage based on their needs.
You can visit and direct your patients to medicareinfocus.com to access helpful resources, including:
- Guidance on how and when to switch between Medicare Advantage and Original Medicare (with or without a Medigap plan)
- A side-by-side cost comparison of Medicare Advantage versus Original Medicare with Medigap
- An overview and FAQs about signing up for Medicare
Medicare Advantage plans terminating in 2026
| In 2026, there are several Medicare Advantage plans that will be discontinued/terminated that may impact your patients. Key considerations for your patients include: | ||||||||||
| • | Automatic enrollment in Original Medicare: If the patient’s Medicare Advantage plan ends, the patient is often automatically enrolled in Original Medicare (Part A and Part B). This ensures the patient maintains health coverage | |||||||||
| • | Special Enrollment Period (SEP): The patient could also qualify for a Special Enrollment Period, during which they may: | |||||||||
|
||||||||||
| • | Patient notification from the health plan: The patient will receive a notice from the Medicare Advantage plan at least 90 days before the plan ends, outlining options and next steps | |||||||||
| • | Coverage continuity: The patient’s current Medicare Advantage plan will remain effective until December 31 of the year it is terminated or discontinued | |||||||||
| • | Medigap Guaranteed Issue Rights: If the patient’s plan is terminated, the patient may have guaranteed issue rights to buy a Medigap policy without medical underwriting. This is only available for a limited time | |||||||||
| • | Keep medical records and bills: Ensure the patient keeps all medical records and bills for reference in case there are any issues during the transition to a new plan | |||||||||
| For additional information, your patients you can use medicareinfocus.com for patient education and additional resources. | ||||||||||
Navigating Insurance: A Guide to Patient Affordability

A guide for HCPs and office staff to understand different payer channels, out-of-pocket considerations, and support services available for patients being treated with EYLEA HD.
Please reach out to your RBM to learn more.
EyeOnAccess Academy
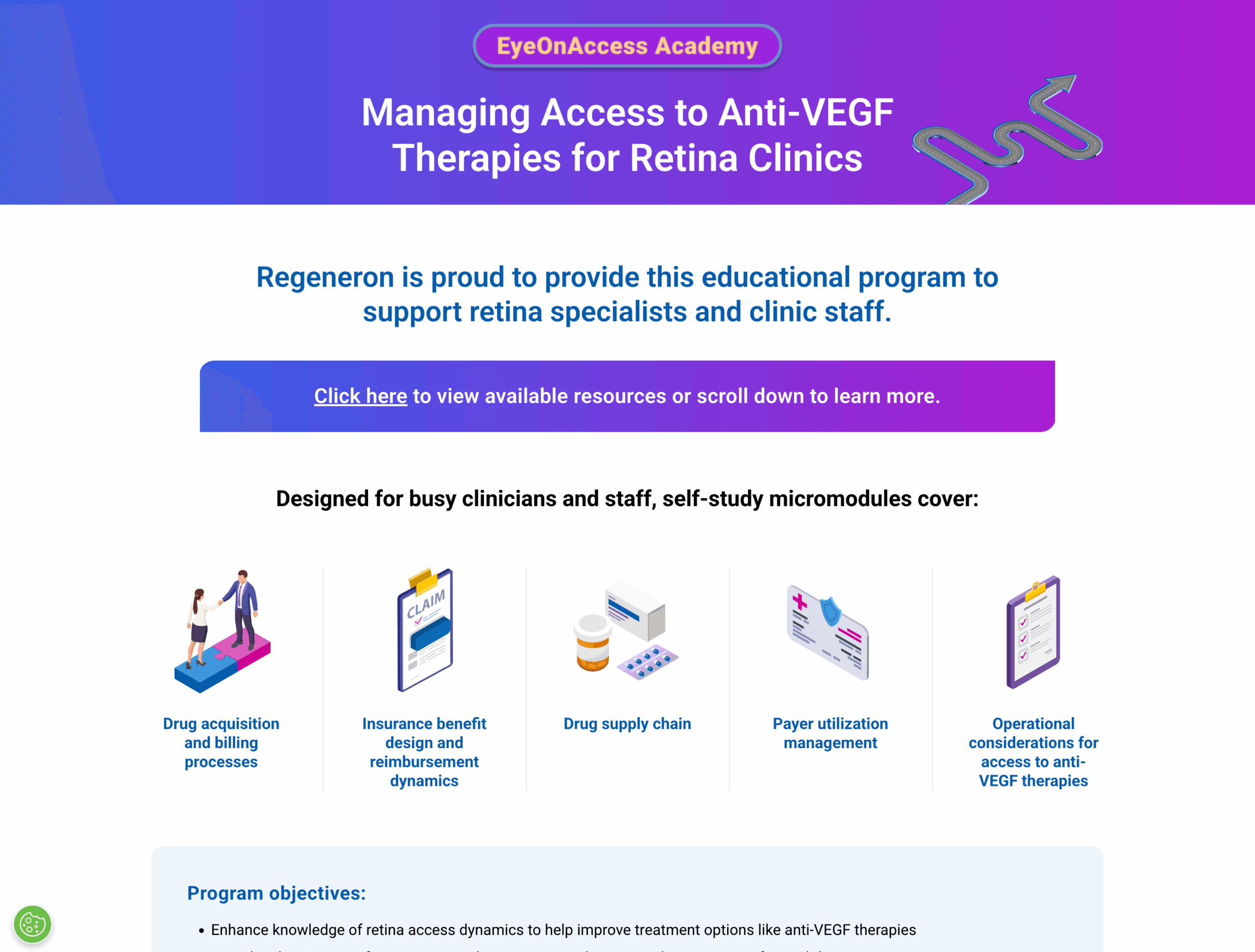
A microsite for HCPs and office staff with self-directed, downloadable modules reviewing drug acquisition and billing processes, insurance benefit design and reimbursement, drug supply chain, payer utilization management, and operational considerations for access to anti-VEGF therapies.
Navigating Payer Challenges (NPC)
| The NPC resource contains information for HCPs and office administrators that may facilitate how to access, submit claims, and seek reimbursement to support their patients’ access to EYLEA. |
| You can also visit NavigatingPayerChallenges.com to: |
|
| We recommend bookmarking the website for your convenience. Updates to the site will be made on a regular basis. |
Reimbursement Information for EYLEA HD1
Vial kit with injection components
- One EYLEA HD 8 mg (0.07 mL of a 114.3 mg/mL solution), single-dose glass vial
-
One 18-gauge x 1 1/2-inch, 5-micron filter needle for withdrawal of the vial contents
- One 30-gauge x 1/2-inch injection needle for intravitreal injection
- One 1-mL syringe for administration
- One Prescribing Information
NDC
- 6175505001
- 61755005001
Note: The product’s NDC has been “zero-filled” to ensure creation of an 11-digit code that meets general billing standards. The zero-fill location is indicated in bold.
Ask your RBM about the EYLEA HD Comprehensive Product Acquisition Brochure, which includes more information on how you can acquire EYLEA HD for your practice.
Permanent J-code for EYLEA HD5
- J0177, Injection, aflibercept hd, 1 mg, is effective for dates of service on or after April 1, 2024
- With the permanent J-code, billing units for EYLEA HD must be reported based on the unit value for the assigned permanent code. EYLEA HD is reported with “8” units of J0177 per treatment
Modifiers required on Medicare FFS claims for EYLEA HD and EYLEA
As of October 1, 2023, claims for drugs from single-dose containers that do not use the required modifiers (JZ and JW) may be returned.6
Reminder: HCPs are required to report the JZ modifier when submitting Medicare FFS claims from all outpatient settings. The JZ modifier is used to attest that no amount of drug was discarded and eligible for payment and that no JW modifier amount is reported (a JW modifier, required since July 1, 2023, is used when indicating that the discarded drug was not administered to a patient). Like the JW modifier, the JZ modifier should only be used for claims that bill for single-dose container drugs as approved by the FDA.
Click here to learn more.
Payment Terms, WAC Discount, and ASP Information for EYLEA HD
Payment terms for EYLEA HD: Effective August 18, 2023
Physician payment terms will be 120 days, subject to distributor qualification.*†
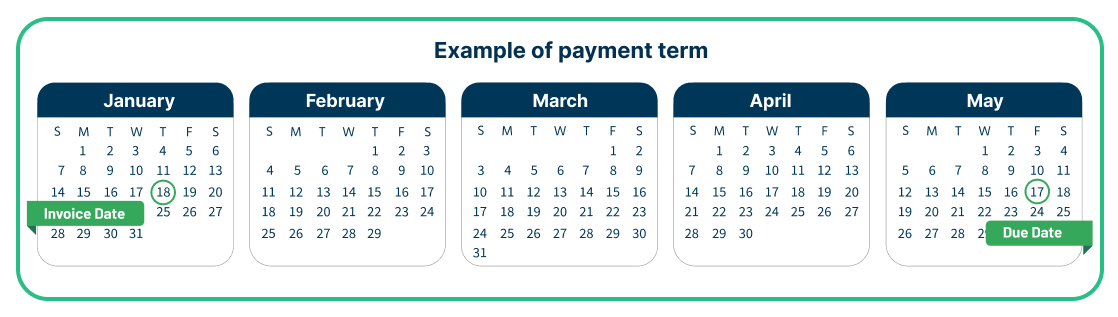
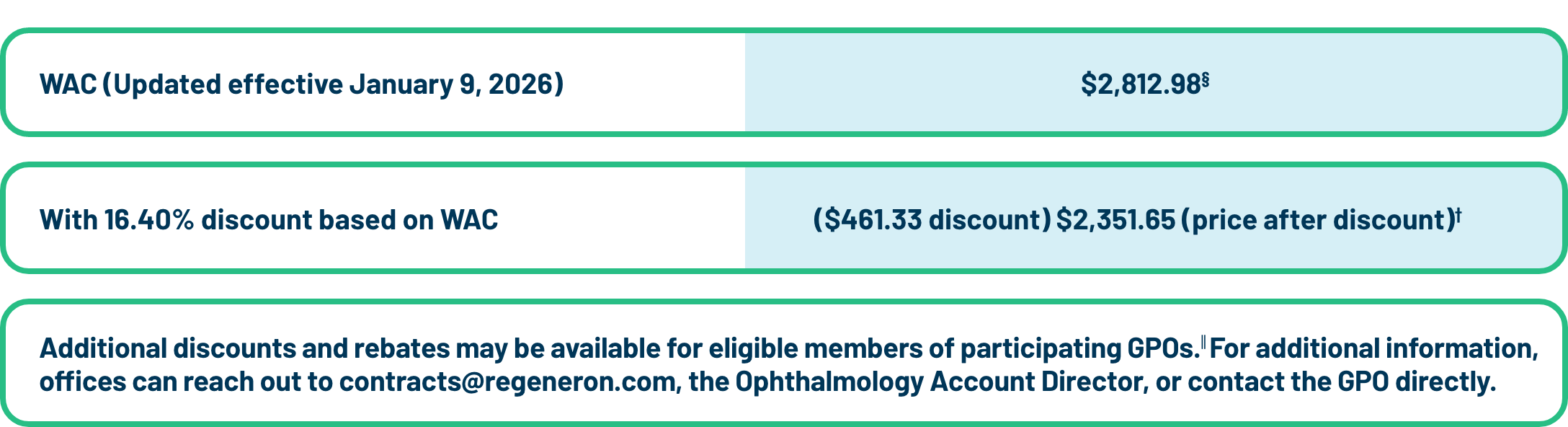
Discount for EYLEA HD: Effective January 9, 2026
The wholesale acquisition cost (WAC) discount is 16.40% on EYLEA HD for community-based physicians in solo or group practices who are treating patients on an outpatient basis (if ordered through authorized distributors).‡

| * | These payment terms are subject to change. Regeneron worked with its authorized distributors of record to apply these terms for EYLEA HD. |
| † | Subject to distributor qualification. Please verify with your distributor the timing and specific dating terms that apply to your office. |
| ‡ | This discount applies only to eligible purchasers ordering from authorized distributors. See EYLEAHDhcp.com for additional discount terms. The EYLEA HD WAC discount is subject to change. |
| § | WAC serves as the list price for EYLEA HD. It does not necessarily reflect the end price paid by purchasers. |
| ∥ | Rebates are subject to additional terms and conditions, including eligibility criteria. Please contact your Regeneron Ophthalmology Account Director for additional information. |
| The discount and rebates described herein are intended to constitute a discount as such term is defined in 42 U.S.C. § 1320a-7b(b)(3) and 42 C.F.R. § 1001.952(h). As such, participating physicians have an obligation to comply with all retention, disclosure, and reporting requirements regarding the value of any discounts or rebates paid or received, including those described herein, pursuant to 42 U.S.C. § 1320a-7b(b)(3) and 42 C.F.R. § 1001.952(h) and all other applicable federal and state laws and regulations. | |
| The EYLEA HD WAC discount is an independent and non-contingent offering specific to EYLEA HD. |
Participating GPOs and Authorized Specialty Distributors
GPOs:
Acuity; AllyRetina¶; IPN; Onmark; Matrix
Specialty Distributors:
Besse Medical; BioCareSD¶; CuraScript SD Specialty Distribution; McKesson Specialty Health; McKesson Plasma & Biologics for Hospitals; Metro Medical (A Cardinal Health Company); and Cardinal Health Specialty Pharmaceutical Distribution for Hospitals
Contact one of our authorized distributors and receive EYLEA HD on the next business day. Orders received prior to 7 PM Eastern Time Monday–Thursday are typically processed on the same day and scheduled for delivery the next business day. Orders received on Friday will typically be delivered the following Monday.
Regeneron does not recommend the use of a particular authorized distributor or GPO.
¶GPO and specialty distributor added as of October 1, 2024.
ASP for EYLEA HD
Effective as of January 1, 2026, the ASP for EYLEA HD in Q1 2026 is $2,316.87.
Access News Roundup
Access-related news affecting the retina care landscape
A CMS claims system issue is causing denials for Medicare and Medicare Advantage claims related to treating Wet AMD and geographic atrophy (GA) when both treatments are given within 25-28 days. The first treatment is typically reimbursed, but the second drug and injection code are being denied.
ASRS and AAO are working with CMS to remove the edit, and retina practices are encouraged to appeal, arguing that Wet AMD and GA are distinct diseases treated with different drugs, all following FDA guidelines.7
Congress passed a bill that includes a 2.5% increase in Medicare physician payments for 2026, with no changes to payment levels through 2025 or after 2026. Starting in 2027, the bill introduces significant changes to Medicaid eligibility and low-income subsidies, though physician reimbursement under Medicaid remains unchanged.8
Reform for Medicare Advantage Policies
CMS released its 2025 Medicare Advantage and Part D plan year final rule, which includes a requirement that Medicare Advantage plans incorporate health equity experts into their utilization management committees and conduct annual health equity assessments of prior authorization policies.9
Educational Opportunities
Practice Administrator Educational Programs
There are several speaker programs available to educate practice administrators and staff on specific topics related to EYLEA HD and EYLEA.
Topics include:
- Access and reimbursement information
- Understanding market access dynamics within your account
- Helpful tips when reviewing payer contracts and documentation
- Processes for benefit verification, prior authorization, medical exceptions, and appeals
Click here to connect with your Regeneron RBM for more information about these speaker programs.
Helpful Documentation Considerations Resource
The Helpful Documentation Considerations guide is available for office use. It provides tips to help ensure proper documentation and a checklist to confirm that the necessary EYLEA HD clinical information is included in all appropriate paperwork to be submitted to payers. It is distributed by RBMs to help physicians facilitate payer coverage and reduce prior authorization denials.

EYLEA4U Information
The EYLEA4U Patient Support Program can provide you with benefit verification support for your patients. All programs within EYLEA4U are available for both EYLEA HD and EYLEA. EYLEA4U forms include both EYLEA and EYLEA HD, which can help streamline the process of enrolling patients who require treatment with EYLEA HD, including those transitioning from EYLEA to EYLEA HD in the EYLEA4U Commercial Copay Card Program or Patient Assistance Program (PAP).
Please note: To receive payments for EYLEA HD through the EYLEA4U Commercial Copay Program, you must complete (one-time only) an EYLEA4U Healthcare Provider Representation Form.
If you haven’t completed this form, please contact your RBM or call EYLEA4U at 1-855-EYLEA4U (1-855-395-3248), Option 4, Monday–Friday, 9 am–8 pm Eastern Time to receive a form today.
The EYLEA4U ePortal has moved to a new platform! Visit E4Ueportal.com to learn more.
EYLEA4U Online Via Tablet-Based Technology*
Tablet-based technology that interfaces with electronic medical record, practice management, and inventory management system technology and can connect patients to all EYLEA4U support services.
- Electronic signature capture for tablet enrollment†
- Electronic submissions in real time
- Electronic enrollment solutions can offer benefit verification accuracy
Contact your Reimbursement Business Manager if you are interested in utilizing the tablet.
| * | Requires the use of a tablet provided by Regeneron’s third-party technology source and a license paid for by the HCP office. |
| † | For tablet only; portal enrollment requires patient signature to be filed. |
CMS Policy Pulse10,11
Responding to criticisms of the current prior authorization (PA) process, CMS has issued a new rule requiring health plans to send PA decisions within three days for urgent requests and within seven days for standard requests.
The new rule, effective in 2026, also requires payers to provide an explanation for every denial of a PA and publicly report their PA metrics.
By 2027, payers will have to implement a PA application programming interface (API) to improve the electronic exchange of health information and automation of the PA process.
CMS estimates the new rule will save approximately $15 billion over 10 years by reducing the administrative burden of PAs and improving patient outcomes.
Additionally, through the Medicare Part B Inflation Rebate Program, CMS may adjust reimbursement amounts for certain Part B drugs when prices outpace inflation, a change that could lower beneficiary OOP costs.12
Stay up-to-date with the latest information on audits from CMS
Current MAC Medical Review List
| MAC | EYLEA J0178 | Medical Review Links |
| CGS J15 Part B | TPE review | Link |
| Palmetto JJ Part B | TPE review | Link |
| Palmetto JM Part B | TPE review | Link |
As of June 2025.
Product Support
Click here for a list of authorized specialty distributors and specialty pharmacy providers for EYLEA HD and EYLEA.
Regeneron does not recommend the use of any particular authorized distributor or specialty pharmacy.
Product return procedure
If EYLEA HD or EYLEA is rendered unusable after purchase, it may be returned to Regeneron and replaced, under certain circumstances. Returns are subject to adherence to Regeneron policies and procedures regarding the return of product and Regeneron’s right, at its sole discretion, to deny replacement when misuse is suspected.
For more information about product returns, click here.
Product Profiles
Explore the dosing flexibility of EYLEA HD in Wet AMD and DME
Discover the data for EYLEA HD for patients with DR and MEfRVO
Resources
Your dedicated RBM can provide support to help your eligible patients access EYLEA HD and EYLEA. They provide support in areas including access and reimbursement, financial assistance, and health plan policy, and can provide you with various educational tools and resources.
Contact your RBM to learn more about the latest tools and resources available for EYLEA HD and EYLEA:
- EYLEA HD and EYLEA Payer Policy Portal
- Enables your practice administrators and office staff to access certain payer policy information for EYLEA HD and EYLEA
- Navigating Insurance: A Guide to Patient Affordability
- Provides guidance for HCPs and office staff to understand different payer channels, out-of-pocket considerations, and support services available for patients being treated with EYLEA HD
- FormTrak Coverage Flash Cards
- Provides coverage status for EYLEA HD and EYLEA by health plan, channel, and location
- Helpful Documentation Considerations Guide
-
Provides your office staff with tips and a checklist to ensure proper documentation for EYLEA HD coverage
Patient Resources
- Medicare in Focus
- Provides patients with information and education on the differences between Original Medicare and Medicare Advantage to support their selection of the most appropriate coverage based on their needs
- RE-Assist®
- An independent charitable foundation resource that helps patients with retinal conditions like Wet AMD, DME, DR, and MEfRVO to identify potential sources of independent, third-party funding for treatment and treatment-related expenses. Access RE-Assist here
RE-Assist is an independent third party not affiliated with Regeneron. Regeneron does not develop the content, is not responsible for the accuracy of the information provided, and does not endorse any particular charitable foundation.
Visit the Resource Center at the EYLEA HD HCP website to download information about EYLEA HD, including:
- Prior Authorization Checklist Flash Card
- Billing and Coding Brochure
- Appeals Letter Overview Brochure
- EYLEA4U Annotated Enrollment Form Brochure
- EYLEA4U Overview Brochure for HCPs
The EYLEA4U Practice Administrator Speaker Program is designed to educate practice administrators and their staff on specific topics related to EYLEA HD and EYLEA. Contact your RBM for the Practice Administrator Speaker Program (RAP) Overview Flash Card to see the wide variety of topics covered through this program.
IMPORTANT SAFETY INFORMATION FOR EYLEA HD AND EYLEA (cont’d)
WARNINGS AND PRECAUTIONS
- Intravitreal injections, including those with aflibercept, have been associated with endophthalmitis and retinal detachments and, more rarely, retinal vasculitis with or without occlusion. Proper aseptic injection technique must always be used when administering EYLEA HD or EYLEA. Patients and/or caregivers should be instructed to report any signs and/or symptoms suggestive of endophthalmitis, retinal detachment, or retinal vasculitis without delay and should be managed appropriately.
- Acute increases in intraocular pressure (IOP) have been seen within 60 minutes of intravitreal injection, including with EYLEA HD and EYLEA. Sustained increases in IOP have also been reported after repeated intravitreal dosing with VEGF inhibitors. IOP and the perfusion of the optic nerve head should be monitored and managed appropriately.
- There is a potential risk of arterial thromboembolic events (ATEs) following intravitreal use of VEGF inhibitors, including EYLEA HD and EYLEA. ATEs are defined as nonfatal stroke, nonfatal myocardial infarction, or vascular death (including deaths of unknown cause).
-
- EYLEA HD: The incidence of reported ATEs in the Wet AMD study from baseline through week 48 was 0.4% (3 out of 673) in the combined group of patients treated with EYLEA HD compared with 1.5% (5 out of 336) in patients treated with EYLEA 2 mg. The incidence in the DME study from baseline to week 48 was 3.1% (15 out of 491) in the combined group of patients treated with EYLEA HD compared with 3.6% (6 out of 167) in patients treated with EYLEA 2 mg. The incidence in the RVO study from baseline to week 36 was 0.5% (3 out of 591) in the combined group of patients treated with EYLEA HD compared with 1.7% (5 out of 301) in patients treated with EYLEA 2 mg.
- EYLEA: The incidence of reported ATEs in Wet AMD studies during the first year was 1.8% (32 out of 1824) in the combined group of patients treated with EYLEA compared with 1.5% (9 out of 595) in patients treated with ranibizumab; through 96 weeks, the incidence was 3.3% (60 out of 1824) in the EYLEA group compared with 3.2% (19 out of 595) in the ranibizumab group. The incidence in the DME studies from baseline to week 52 was 3.3% (19 out of 578) in the combined group of patients treated with EYLEA compared with 2.8% (8 out of 287) in the control group; from baseline to week 100, the incidence was 6.4% (37 out of 578) in the combined group of patients treated with EYLEA compared with 4.2% (12 out of 287) in the control group. There were no reported thromboembolic events in the patients treated with EYLEA in the first six months of the RVO studies.
ADVERSE REACTIONS
- EYLEA HD: The most common adverse reactions (≥3%) reported in patients receiving EYLEA HD were cataract, conjunctival hemorrhage, corneal epithelium defect, intraocular pressure increased, ocular discomfort/eye pain/eye irritation, retinal hemorrhage, vision blurred, vitreous detachment, and vitreous floaters.
- EYLEA: Serious adverse reactions related to the injection procedure have occurred in <0.1% of intravitreal injections with EYLEA including endophthalmitis and retinal detachment. The most common adverse reactions (≥5%) reported in patients receiving EYLEA were conjunctival hemorrhage, eye pain, cataract, vitreous detachment, vitreous floaters, and intraocular pressure increased.
- Patients may experience temporary visual disturbances after an intravitreal injection with EYLEA HD or EYLEA and the associated eye examinations. Advise patients not to drive or use machinery until visual function has recovered sufficiently.
AMD = age-related macular degeneration; AAO = American Academy of Ophthalmology; ASP = average sales price; ASRS = American Society of Retina Specialists; CMS = Centers for Medicare & Medicaid Services; DME = diabetic macular edema; DR = diabetic retinopathy; ERISA = Employee Retirement Income Security Act; FDA = US Food and Drug Administration; HCP = health care provider; GPO = group purchasing organization; MAC = Medicare Administrative Contractor; MEfRVO = macular edema following retinal vein occlusion; NDC = National Drug Code; OOP = out-of-pocket; Q4W = every 4 weeks; RBM = Reimbursement Business Manager; VEGF = vascular endothelial growth factor.
References: 1. EYLEA HD full U.S. Prescribing Information. Regeneron Pharmaceuticals, Inc. November 2025. 2. Centers for Medicare & Medicaid Services. Medicare Benefit Policy Manual, Chapter 15: Covered medical and other health services. Revised April 11, 2025. Accessed July 22, 2025. https://www.cms.gov/regulations-and-guidance/guidance/manuals/downloads/bp102c15.pdf 3. Data on file. Regeneron Pharmaceuticals, Inc. 4. Robeznieks A. New physician “gold card” law will cut prior authorization delays. American Medical Association. Accessed July 22, 2025. https://www.ama-assn.org/practice-management/prior-authorization/new-physician-gold-card-law-will-cut-prior-authorization 5. Centers for Medicare & Medicaid Services. Centers for Medicare & Medicaid Services (CMS) Healthcare Common Procedure Coding System (HCPCS) application summaries and coding recommendations: fourth quarter, 2023 HCPCS coding cycle. Accessed July 22, 2025. https://www.cms.gov/files/document/2023-hcpcs-application-summary-quarter-4-2023-drugs-and-biologicals-updated-04/25/2024.pdf 6. Medicare program: discarded drugs and biologicals—JW modifier and JZ modifier policy frequently asked questions. Accessed July 22, 2025. https://www.cms.gov/medicare/medicare-fee-for-service-payment/hospitaloutpatientpps/downloads/jw-modifier-faqs.pdf 7. American Society of Retina Specialists. ASRS seeks to remove CMS edit for billing Wet and Dry AMD treatments in the same month. February 18, 2025. Accessed July 22, 2025. https://www.asrs.org/advocacy/updates/10407/asrs-seeks-to-remove-cms-edit-for-billing-wet-and-dry-amd-treatments-in-the-same-month 8. American Society of Retina Specialists. Physician payment update enacted in Republican agenda bill. July 8, 2025. Accessed July 30, 2025. https://www.asrs.org/advocacy/updates/10436/physician-payment-update-enacted-in-republican-agenda-bill 9. Centers for Medicare & Medicaid Services. Contract year 2025 Medicare Advantage and Part D final rule (CMS-4205-F). Accessed July 22, 2025. https://www.cms.gov/newsroom/fact-sheets/contract-year-2025-medicare-advantage-and-part-d-final-rule-cms-4205-f 10. CMS finalizes rule to expand access to health information and improve the prior authorization process. News release. Centers for Medicare & Medicaid Services. Accessed July 22, 2025. https://www.cms.gov/newsroom/press-releases/cms-finalizes-rule-expand-access-health-information-and-improve-prior-authorization-process 11. Tong N. CMS finalizes rule setting prior authorization deadlines for payers. Accessed July 22, 2025. https://www.fiercehealthcare.com/payers/cms-introduce-prior-authorization-deadlines-payers 12. Centers for Medicare & Medicaid Services. Medicare inflation rebate program. Accessed December 8, 2025. https://www.cms.gov/priorities/medicare-prescription-drug-affordability/overview/medicare-inflation-rebate-program
EYLEA HD, EYLEA, and EYLEA4U are registered trademarks of Regeneron Pharmaceuticals, Inc.
All other trademarks are property of their respective owners.